
Induction of 5-lipoxygenase by 4-hydroxynonenal via Nitric Oxide Generation in Vascular Smooth Muscle Cells



Abstract
The induction of 5-lipoxygenase (5-LO) by 4-hydroxynonenal (HNE) is of significant interest for understanding the mechanisms underlying the homeostasis of the cellular redox balance, leading to retinal dysfunction. However, the roles of these mediators in vascular smooth muscle cells (VSMC) remain unclear. This study investigated whether the induction of 5-LO by HNE was mediated by nitric oxide (NO) generation in VSMC.
A10 cells were preincubated in DMEM containing HNE after preincubation with NO inhibitors. The gene expression levels of 5-LO were analyzed by western blotting and reverse transcriptase-polymerase chain reaction. Intracellular NO formation was measured by fluorescence using 4,5-diaminofluorescein as a specific NO indicator using flow cytometry.
The fluorescence profiles increased in a time-dependent manner in cells treated with 1mM HNE. These data indicate that HNE induced NO generation in VSMC. NO inhibitors prevent HNE-induced NO generation and 5-LO expression. In addition, the results showed the upregulation of 5-LO mRNA levels by HNE. This suggests that HNE plays a role in regulating 5-LO at the transcriptional level without the possibility of post-translational modification.
These findings suggest that HNE-induced oxidative stress, including NO generation, is an important risk factor for retinopathy development via 5-LO gene expression in VSMC. The induction of 5-LO by HNE in VSMC is mediated by NO generation, leading to the deterioration of vasculature homeostasis and subsequent retinal dysfunction, such as retinopathy.
초록
4-hydroxynonenal (HNE)에 의한 5-lipoxygenase (LO)의 유도에 대해 망막기능장에 이르는 세포내 산화환원균형의 항상성 기전을 이해하는 것이 중요한 관심사이다. 그러나 혈관평활근세포(VSMC)에서 이러한 매개체의 역할에 대해서는 아직 이해해야 할 부분이 많이 남아 있다. 이 연구는 VSMC에서 HNE에 의한 LO의 유도가 산화질소(NO)의 생성에 의해 매개되는지를 조사하는 것이 목적이다.
쥐 대동맥 평활근세포주인 A10 세포를 NO 억제제로 전 처리한 후 HNE를 함유하는 DMEM(0.5% FBS)에서 배양하였다. 단백질 발현은 Western blot과 RT-PCR로 분석하였다. 세포내 NO의 생성은 특이적 NO 지표인 4,5-Diaminofluorescein (DAF-2)를 사용하여 Flow Cytometry로 측정하였다.
1 mM HNE를 처리한세포에서 시간의존적으로 형광의 정도가 증가하였다. 이러한 관찰은 VSMC에서 HNE에 의해 NO의 생성이 유도되었다는 것을 의미한다. NO 저해제는 HNE에 의해 유도되는 NO의 생성과 5-LO의 발현을 억제했다. 또한 HNE는 5-LO의 mRNA의 생성을 증가시켰다. 이러한 결과는 HNE가 번역 후 변형없이 전사 수준에서 5-LO 유전자를 조절하는 것을 시사한다.
본 연구의 결과는 혈관평활근세포에서 NO 생성과 같이 HNE가 유발하는 산화스트레스가 5-LO 유전자 발현과 관련된 망막병증의 발달에 중요한 위험 인자임을 시사한다. HNE에 의한 5-LO의 유도는 혈관계 항상성을 악화시켜 망막병증을 포함하는 망막기능장애를 초래하는 것으로 사료된다.
Keywords:
5-Lipoxygenase, 4-Hydroxynonenal, NO, Retinopathy, VSMC키워드:
5-리폭시제네이즈, 4-하이드록시노네날, 산화질소, 망막병증, 혈관평활근세포Introduction
Retinopathy including diabetic retinopathy (DR) is among the leading cause of new-onset blindness in adults.[1] Retinopathy is a condition that affects the blood vessels in the retina of the eye, which is the part of the eye responsible for vision. When blood vessels become damaged or diseased, it can lead to retinopathy.[2] Several studies have investigated the relationship between retinopathy and the vasculature of the retina.[3,4] DR is a complication of diabetes and occurs when high blood sugar levels damage the blood vessels in the retina. Over time, these damaged blood vessels can leak fluid and blood, which can cause swelling and vision loss.[2]
Lipid peroxidation end product, 4-hydroxynonenal (HNE) can damage the cells that make up the retina, leading to inflammation and oxidative stress.[5] Kapphahn et al. reported that oxidative stress-induced lipid peroxidation has been implicated in the pathogenesis of many degenerative ocular diseases including cataractogenesis, age related macular degeneration, and retinopathy.[6,7] The formation of relatively higher amounts of Lipid peroxidation products including relatively stable and toxic electrophiles such as 4-HNE that may contribute to retinopathy.[8]
This can cause changes in the structure and function of the retinal blood vessels, leading to vision problems. In addition to damaging the retina directly, 4-HNE can also contribute to the development of other risk factors for retinopathy. 4-HNE is thought to influence the development and progression of retinopathy. Understanding the role of 4-HNE in retinopathy may lead to the development of new treatments that can slow or even reverse the damage caused by this disease.
4-HNE is a highly reactive lipid peroxidation product that is formed by oxidative stress in cells. It has been shown to induce the expression of various enzymes and proteins involved in inflammation, including lipoxygenase (LO). LO is an enzyme involved in the biosynthesis of leukotrienes, which are lipid mediators that play a role in various inflammatory diseases.[9] In vascular disease, vascular smooth muscle cell (VSMC) undergo a phenotypic switch from a contractile to a synthetic state, leading to abnormal proliferation and migration. This process is mediated in part by the activation of growth factor receptors and the production of reactive oxygen species. LO-derived leukotrienes are also involved in this process by promoting VSMC proliferation and migration, as well as by induction inflammation and oxidative stress. [10]
It is accumulated evidence which involvement of nitric oxide (NO) in the pathogenesis of retinopathy.[11] Hyperglycemia and other stress stimuli trigger superoxide production, which reacts excessively and rapidly with NO to produce peroxynitrite, potent oxidizing agent.[12] Changes that contribute to oxidative and nitrifying stress, such as increased production of NO and superoxide, altered expression of various isoforms of nitric oxide synthase, or altered expression of endogenous antioxidant systems, have been implicated in the mechanism by which this ocular disease develops.[13]
Research into the role of NO in retinopathy is ongoing, and there is still much to learn about how this molecule affects the health of the retina. Our previous findings revealed that depolarization of VSMC induced by HNE is related to 5-LO.[14] LO is involved in chronic vascular pathologies, including diabetic retinopathy and age-related macular degeneration.[15,16] The aim of the present further study that action mechanism of 4-HNE on induction of 5-LO in VSMCs. In view of our results we can conclude that 4-HNE-induced oxidative stress is an important risk factor in the development of retinopathy via 5-LO induction. The induction of 5-LO by 4-HNE is therefore significant interest in understanding the mechanisms underlying homeostasis of cellular redox balance leading to retinal dysfunction.
Materials and Methods
1. Culture conditions and HNE treatments
The A10 cells, rat aortic smooth muscle cell line, were obtained from American type culture collection (American type culture collection, USA) and grown in Dulbecco's modified Eagle's medium (DMEM) (Sigma Aldrich, USA) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air.
For all experiments, cells were seeded at a density of 4×104/well into 12-well microplates with DMEM containing 10% FBS and supplements. Cells were allowed to adhere in dish overnight, and then the culture medium was replaced with fresh DMEM (0.5% FBS) containing HNE after pre-incubated for 30 min with various inhibitors. A commercial HNE (purity > 98%) was obtained from Cayman Chemical Inc. (Cayman Chemical Inc, USA), and working solutions of HNE were made in phosphate buffered saline (PBS) immediately before use. Working solutions of HNE (< 0.1% ethanol) were made in PBS immediately before use.
2. Assay of NO formation
Intracellular nitric oxide (NO) formation was measured by fluorescence using 4,5-Diaminofluorescein (DAF-2). 4,5-Diaminofluorescein (DAF-2) as a specific NO indicator selectively traps NO between two amino groups in its molecule, and yields triazolofluorescein, which emits green fluorescence when excited at 490-495 nm.[17] A stock solution of 1 mg DAF-2 in 0.55 ml dimethyl sulfoxide was stored at −20°C. The working solution with 0.5 μg DAF-2 was diluted with 50 mM phosphate buffer (pH 7.4) purged with nitrogen. A NO inhibitors, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, NO scavenger) 50 mM and nitro-l-arginine methyl ester (l-NAME, NO synthase inhibitor) were added to a 12-well microplate. The fluorescence intensity was dependent on the amount of NO trapped by DAF-2. The cells grown in 12-well plates were loaded with 10 μM DCFH-DA for 30 min, and then incubated with HNE for 30 min at 37°C. Reactions were stopped by aspiration and cells were trypsinized, collected on ice, and analyzed on Flow Cytometry. Fluorescence intensity was measured for 10,000 cells in each sample. Results were obtained as histogram plots of cell number versus fluorescence intensity (FL-1), and the mean fluorescence for each sample within an experiment was analyzed as% of total cells (BD FACSLyric, BD Biosciences, USA).
3. Protein analysis by Western blot analysis
Cells were harvested, washed twice with ice-cold NaCl/P i, and lysed in a TNN buffer (50 mM Tris/HCl, pH 8.0, 120 mM sodium chloride, 0.5% Nonidet P-40) supplemented with protease inhibitors (2 mg/mL-1 aprotinin, 2 mg/mL-1 leupeptin, 100 mg/mL-1 phenylmethanesulfonyl fluoride, 5 lg/mL-1 pepstatin, 1 m M dithiothreitol) and phosphates inhibitors (20 mM NaF, 2 m M Na3VO4) for 1 h on ice, vortexing every 10 min. Lysates were centrifuged at 8000 g for 30 min to remove insoluble material. Protein concentration was determined by the Lowry method using BSA as standard. Equal amounts of protein were separated on 10-15% SDS/PAGE gels. The gels were subsequently transferred onto a nitrocellulose membrane (BIO-RAD, Korea).
4. Assay on Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
(a) Reverse Transcription: The first strand cDNA was synthesized from 2 μg of total RNA. DEPC-treated water and 250 ng random primer were added, incubated for 5 min at 75°C, and incubated on ice for 5 min. Aliquots of 2 μl 0.1 M DTT, 4 μl 5 × buffer, 4 μl 2.5 mM dNTP, 100 U reverse transcriptase and 16.5 U of RNase inhibitor were added and incubated for 2 hr at 37°C. The reaction was stopped by boiling for 2 min at 100°C, and cDNA was stored at −20°C until use.
(b) Polymerase Chain Reaction: The primer pairs for rat mtDNA were as follows: sense, 5'-TAG GAC AGC CAG GCG CAC TCC-3'; anti-sense, 5'-TTA GTG GGG TGG ATA ATG GAT CGG-3'. Rat 18S ribosomal DNA primers were used as control for the efficiency of mtDNA synthesis in each sample. The primer pairs were as follows: sense, 5'-GGA CCA GAG GCA AAG CAT TTG CC-3'; anti-sense, 5'-TCA ATC TCG GGT GGC TGA ACG C-3'. Reaction conditions consisted of 22 cycles for rat mtDNA and rat 18S ribosomal DNA at 94°C for 15 sec and 61°C for 6 min, followed by a final extension of 10 min at 72°C for 10 min. Electrophoresis was performed in 0.8% agarose gel. The integrity of RT-PCR products was monitored by ethidium bromide staining under U.V. transilluminator.
5. Statistics
All data represent the mean ± S.E.M. Flow cytometry data are from experiments conducted using triplicate incubations. Statistical significance of difference between untreated control and treated groups was analyzed by two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test (Sigma Stat 2.0, Jandel Scientific, Chicago, IL). Differences were considered statistically significant at p<0.05.
Results and Discussion
1. Induction of NO generation by 4-HNE in time-dependent manner
Lipid peroxidation and its reactive byproducts, such as HNE, have been reported to cause redox-related degenerative processes, including vascular dysfunction.[18,19] 4-HNE-induced oxidative stress plays a significant role in the pathogenesis of various of retinopathies, including age-related macular degeneration, DR, and retinal degeneration. (20) To assess the HNE-induced VSMC activation contained oxidative stress, we analyzed NO generation which is producing the potent oxidant peroxynitrite. We monitored changes in NO production by loading with DAF-2. The fluorescence intensity analyzed using FACS. As shown in Fig. 1, the quantity of fluorescent profiles increased with time-dependent manner (Fig. 1B) in the cells treated with 1mM HNE. These observations indicate that HNE-induced NO generation in VSMC.
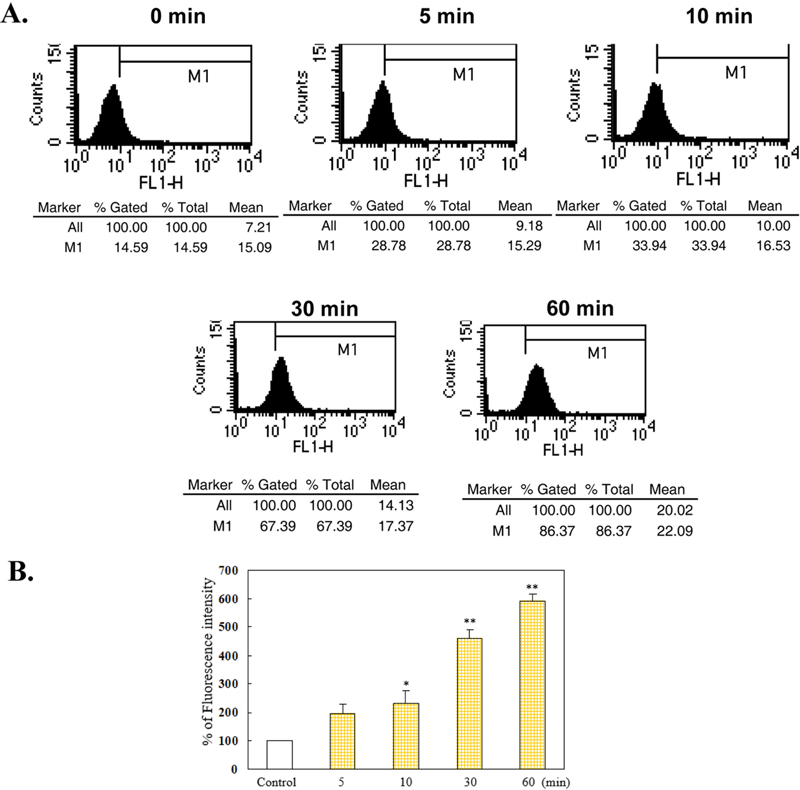
Induction of NO generation by HNE in a time-dependent manner.Cells were incubated with 1 μM HNE for 5–60 min and then treated with DAF-2. Data are presented as the DAF-2 fluorescence intensity using a FACS histogram. A. Results were obtained as histograms of cell number versus fluorescence intensity (FL-1), and the mean fluorescence for each sample within an experiment is analyzed as the percentage of total cells. B. Graph showing NO production induced by HNE as a percentage of the control. The results represent the mean±S.E.M. of three independent experiments. Statistical significance: *p<0.05 vs. control; **p<0.01 vs. vehicle
2. Protective effects of NO inhibitor against HNE-induced NO formation
We monitored protection of NO inhibitors against HNE-induced NO formation. As shown in Fig. 2, NO inhibitors protected the NO generation in the cells treated with 1 mM HNE. Pretreatment with NO inhibitor, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, NO scavenger) 50 mM and nitro-l-arginine methyl ester (l-NAME, NO inhibitor) prevented HNE-induced NO generation. These observations indicate that HNE induce NO generation in VSMC. This result was confirmed in an experiment using inhibitors.
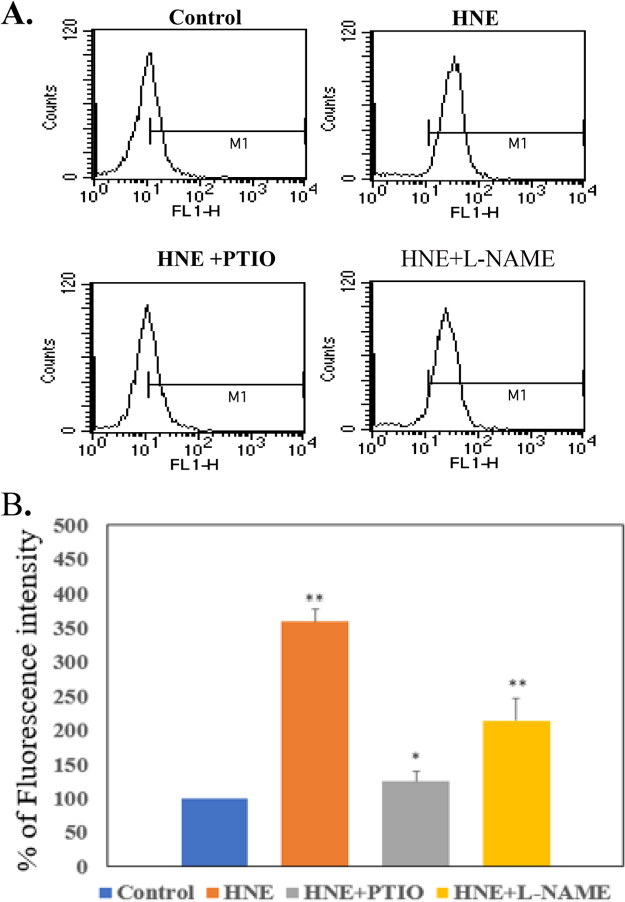
Protective effects of LO inhibitors against HNE-induced NO formation.The cells were incubated with 1 μM HNE for 4 h after pre-treatment for 30 min with LO inhibitors, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO, NO scavenger) 50 μM and nitro-l-arginine methyl ester (L-NAME, NO synthase inhibitor) 10 M, with DAF-2 and analyzed using FACS. A. Representative histogram plots of cell numbers versus fluorescence intensity (FL-1). B. Data represent the percentage of DCF fluorescence intensity relative to the control. The results represent the mean ± S.E.M. of three independent experiments. Statistical significance: *p<0.05 vs. control; **p<0.01 vs. control
Several studies have suggested that HNE induced oxidative stress in the vasculature, which can have both beneficial and harmful effects. In the vasculature, 4-HNE-induced oxidative stress can activate the inducible nitric oxide synthase (iNOS) pathway, leading to the NO generation.[21] Excessive NO generation NO can also be detrimental to the vasculature. In the presence of O2-, NO can react to form peroxynitrite (NOO-), a highly reactive oxidant that can cause oxidative damage to various molecules including lipids, proteins, and DNA. This can lead to endothelial dysfunction, inflammation, and vascular remodeling, which are all hallmarks of vascular disease.[22,23] Taken together, actions of 4-HNE-induced NO production in the vasculature are complex and context- dependent. Although NO production may have short-term beneficial effects in response to oxidative stress, excessive NO production may lead to oxidative damage in the long term and contribute to vascular disease.
3. Suppression of NO scavenger on the HNE-induced 5-LO gene expression.
In our previous study, HNE-induced 5-LO expression was found to be associated with mitochondrial depolarization leading to apoptosis.[14] Studies have shown that 5-LO inhibitors prevented retinal damage in diabetic retinopathy.[24] Therefore, we further investigated the mechanism by which HNE affects the expression of 5-LO in this study. We tested whether NO inhibitor block HNE-induced 5-LO expression. At 1 mM HNE, 5-LO gene expression was increased (Fig. 3) but was decreased by NO inhibitor, PTIO. In order to study the action of NO on 5-LO gene expression induced by HNE, inhibitors were tested to block 5-LO protein level. The results showed that HNE and SNAP, NO donor, induced 5-LO expression, ultimately leading to VSMC dysfunction (Fig 3). Nonspecific ROS scavenger, NAC did not completely prevent HNE-induced 5-LO expression. The increased protein level of 5-LO by SNAP was blocked when treated with PTIO indicating that NO generation is closely involved in HNE-derived 5-LO expression, thereby leading to VSMC activation. The role of HNE in the activation of VSMC was announced in our previous paper.[25]
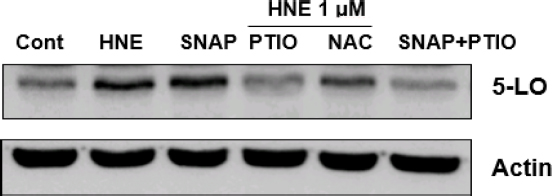
Suppression of NO scavenger on the HNE-induced 5-LO gene expression.The cells were incubated with 1 μM HNE for 24 h after pre-treatment with the inhibitors for 30 min. 5-LO proteins were analyzed by western blotting. Data are representative of at least three independent experiments. Upregulation of 5-LO gene expression by HNE, SNAP, and NO donors. PTIO suppresses 5-LO expression induced by HNE or SNAP. PTIO: NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline -1-oxyl-3-oxide 50 μM; SNAP: NO donor, S-nitroso-N-acetylpenicillamine 1 mM; NAC: ROS scavenger, N-acetylcysteine 500 μM
These findings suggest that NO mediates HNE-induced 5-LO expression and activation by NO excessive production, resulting in deterioration of vasculature homeostasis and subsequent vascular dysfunction such as retinal disease. Based on these results, it is considered that 5-LO expression induced by HNE is closely related NO generation. In our study, it was shown that HNE induces the 5-LO expression and this process is closely related to the production of NO.
4. Up-regulation of 5-LO mRNA induced by HNE
To further characterize altered 5-LO, changes in the RNA were analyzed by RT-PCR with the 5-LO specific probe. Interestingly, the results showed up-regulation of 5-LO mRNA level by HNE (Fig. 4). These results suggest HNE plays a role on gene modulation of 5-LO in transcriptional levels without possibility of post-translation modifications.
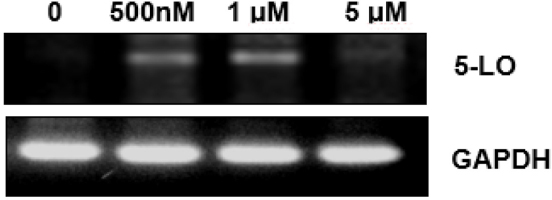
Upregulation of VSMC 5-LO mRNA induced by HNE.RT-PCR was performed to determine the levels of 5-LO mRNA in VSMC. GAPDH was used as a positive control. Upregulation of 5-LO mRNA levels in a dose-dependent manner. Data are representative of at least three independent experiments.
Vascular diseases for the eye, such as diabetic retinopathy, retinal vein occlusion, and age-related macular degeneration, which increase in population with aging, are major causes of serious visual impairment. HNE is a reactive aldehyde closely related to aging and increases with aging.[24] Retinopathy is caused by HNE-induced oxygen exposure in several retinal diseases, including retinopathy of prematurity, diabetic retinopathy, macular degeneration and central vein occlusion.[26] The enzyme LO is known to be an enzyme that participates in mediation the inflammatory response caused by retinal changes in diabetic retinopathy.[27,28]
However, NO-related HNE’s effect to induce vasculature dysfunction through the regulation of 5-LO is not yet well described. Thus, it is important to define the role of HNE in VSMC activation in relation to 5-LO gene expression. Our study suggests that 4-HNE plays an important role in the production of NO during this activation mechanism. Although more preliminary studies are needed, these studies can serve as a starting point for investigating the complex biological mechanisms involved in 4-HNE, NO, 5-LO, and VSMC. Thake together, our data have shown that the induction of 5-LO by 4-HNE in VSMCs is mediated by NO generation leading to vascular dysfunction such as retinopathy.
Conclusion
Retinal disease is closely related to vision, and vascular abnormalities are also one of the important causes of retinal disease. Therefore, it is very important to study the function of HNE in vascular smooth muscle cells, which causes serious problems in blood vessels. Taken together, these results suggest that induction of 5-lipoxygenase by 4-HNE is via NO generation in VSMC, leading to the deterioration of vasculature homeostasis and subsequent vascular dysfunction such as retinopathy.
Acknowledgments
This work was researched with a grant from the Suseong University research grant in 2022.
References
-
Agriodimos G, Gallos P, Tasoulis S, et al. An online information tool for diabetic retinopathy. Stud Health Technol Inform. 2021;287:167-168.
[https://doi.org/10.3233/SHTI210840]

-
Crawford TN, Alfaro DV 3rd, Kerrison JB, et al. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8-13.
[https://doi.org/10.2174/157339909787314149]

-
Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102-180.
[https://doi.org/10.1016/j.preteyeres.2012.08.004]

-
Nakano A, Kondo R, Kaneko Y, et al. Changes in components of the neurovascular unit in the retina in a rat model of retinopathy of prematurity. Cell Tissue Res. 2020;379(3):473-486.
[https://doi.org/10.1007/s00441-019-03112-9]

-
Vatsyayan R, Chaudhary P, Sharma A, et al. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92(2):147-154.
[https://doi.org/10.1016/j.exer.2010.11.010]

-
Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375-413.
[https://doi.org/10.1016/0039-6257(88)90052-5]

-
Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. J Diabetes Res. 2007;2007:43603.
[https://doi.org/10.1155/2007/43603]

-
Kapphahn RJ, Giwa BM, Berg KM, et al. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp Eye Res. 2006;83(1):165-175.
[https://doi.org/10.1016/j.exer.2005.11.017]

-
Chen SH, Fahmi H, Shi Q, et al. Regulation of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase-activating protein/5-lipoxygenase by 4-hydroxynonenal in human osteoarthritic chondrocytes. Arthritis Res Ther. 2010;12(1):R21.
[https://doi.org/10.1186/ar2926]

-
Natarajan R, Rosdahl J, Gonzales N, et al. Regulation of 12-lipoxygenase by cytokines in vascular smooth muscle cells. Hypertension. 1997;30(4):873-879.
[https://doi.org/10.1161/01.hyp.30.4.873]

-
Sharma S, Saxena S, Srivastav K, et al. Nitric oxide and oxidative stress is associated with severity of diabetic retinopathy and retinal structural alterations. Clin Exp Ophthalmol. 2015;43(5):429-436.
[https://doi.org/10.1111/ceo.12506]

-
González P, Lozano P, Ros G, et al. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. 2023;24(11):9352.
[https://doi.org/10.3390/ijms24119352]

-
Opatrilova R, Kubatka P, Caprnda M, et al. Nitric oxide in the pathophysiology of retinopathy: evidences from preclinical and clinical researches. Acta Ophthalmol. 2018;96(3):222-231.
[https://doi.org/10.1111/aos.13384]

-
Lee J. 5-Lipoxygenase mediates vascular smooth muscle cell depolarization induced by 4-hydroxynonenal. J Korean Ophthalmic Opt Soc. 2022;27(1):89-95.
[https://doi.org/10.14479/jkoos.2021.27.1.89]

-
Sapieha P, Stahl A, Chen J, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12.
[https://doi.org/10.1126/scitranslmed.3001571]

-
Wang MH, Hsiao G, Al-Shabrawey M. Eicosanoids and oxidative stress in diabetic retinopathy. Antioxidants. 2020;9(6):520-532.
[https://doi.org/10.3390/antiox9060520]

-
Sutherland H, Khundkar R, Zolle O, et al. A fluorescence-based method for measuring nitric oxide in extracts of skeletal muscle. Nitric Oxide. 2001;5(5):475-481.
[https://doi.org/10.1006/niox.2001.0374]

-
Lee JY, Je JH, Kim DH, et al. Induction of endothelial apoptosis by 4-hydroxyhexenal. Eur J Biochem. 2004;271(7):1339-1347.
[https://doi.org/10.1111/j.1432-1033.2004.04042.x]

-
Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279(12):11789-11797.
[https://doi.org/10.1074/jbc.M311184200]

-
Tanito M, Haniu H, Elliott MH, et al. Identification of 4-hydroxynonenal-modified retinal proteins induced by photooxi-dative stress prior to retinal degeneration. Free Radic Biol Med. 2006;41(12):1847-1859.
[https://doi.org/10.1016/j.freeradbiomed.2006.09.012]

-
Lee JY, Je JH, Jung KJ, el al. Induction of endothelial iNOS by 4-hydroxyhexenal through NF-kappaB activation. Free Radic Biol Med. 2004;37(4):539-548.
[https://doi.org/10.1016/j.freeradbiomed.2004.05.011]

-
Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27(9):1877-1885.
[https://doi.org/10.1161/ATVBAHA.107.142943]

-
Abdulle AE, Diercks GFH, Feelisch M, et al. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front Physiol. 2018;9:1177.
[https://doi.org/10.3389/fphys.2018.01177]

-
Moreau R, Heath SHD, Doneanu CE, et al. Age-related increase in 4-hydroxynonenal adduction to rat heart alpha-ketoglutarate dehydrogenase does not cause loss of its catalytic activity. Antioxid Redox Signal. 2003;5(5):517-527.
[https://doi.org/10.1089/152308603770310167]

-
Lee JY. The role of 4-hydroxynenal for activation of vascular smooth muscle cell. Korean J Vis Sci. 2019;21(2):281-289.
[https://doi.org/10.17337/JMBI.2019.21.2.281]

-
Chapple SJ, Cheng X, Mann GE. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1(1):319-331.
[https://doi.org/10.1016/j.redox.2013.04.001]

-
Al-Shabrawey M, Mussell R, Kahook K, et al. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011;60(2):614-624.
[https://doi.org/10.2337/db10-0008]

-
Gubitosi-Klug RA, Talahalli R, Du Y, et al. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57(5):1387-1393.
[https://doi.org/10.2337/db07-1217]
